Applications of gene therapy in clinical trials
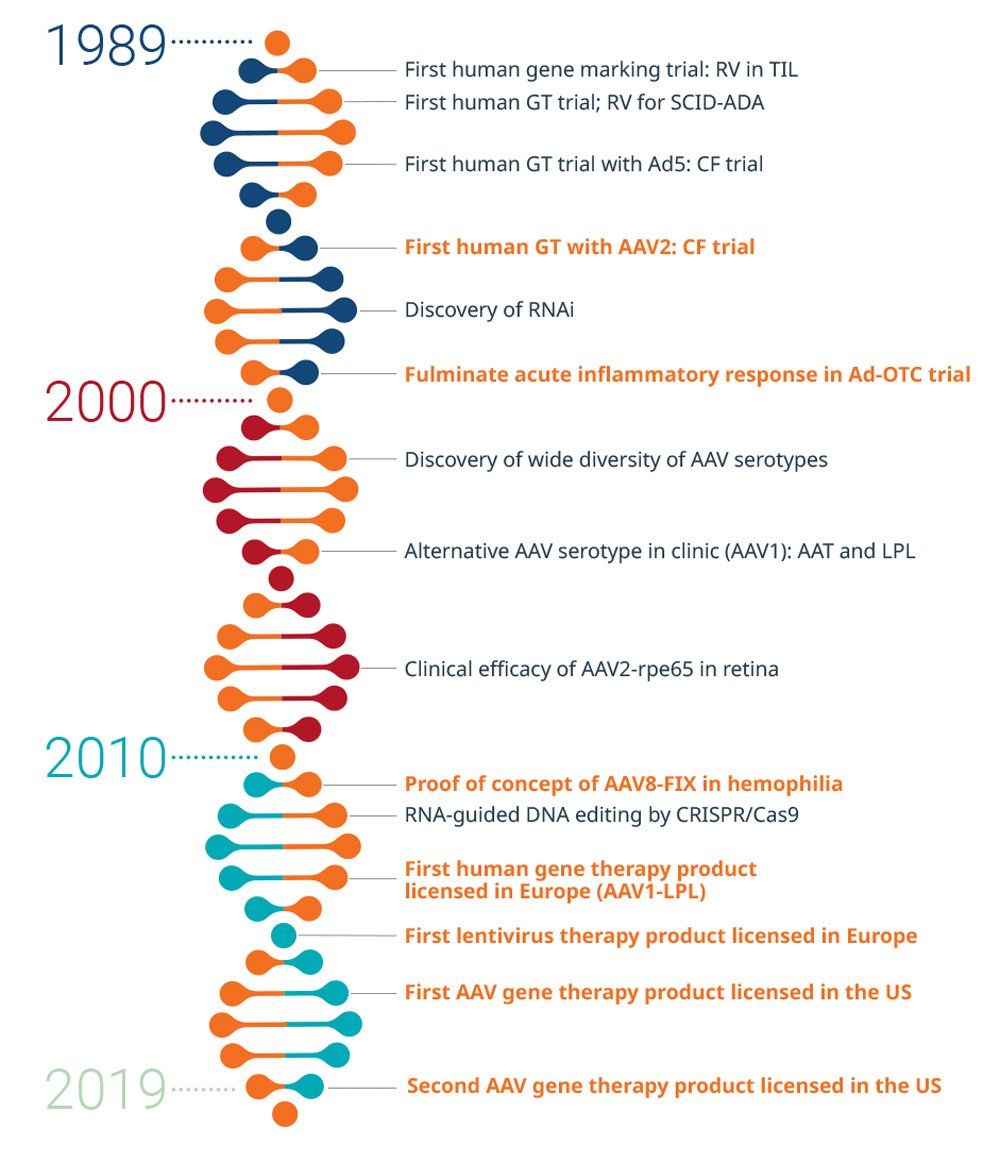
History of gene therapy
History of gene therapy
Efforts to develop AAV-based gene therapy to a stage where it may safely treat genetic diseases has taken 30 years.1
In the late 1980s and early 1990s, progress with AAV-based gene therapy in animals and humans was pushed forward by the Cystic Fibrosis Foundation. In 1995, the first human with cystic fibrosis received AAV-based gene therapy. Within 10 years, trials using AAV-based gene therapy for other genetic diseases, such as hemophilia B, were underway.2
Scalable methods for producing high-quality products are now available. As of January 2020, the FDA has issued six final guidance documents to support innovation in the development of gene therapy products; and as of July 2020, there are two FDA-approved AAV-based gene therapies in the United States.3,4
AAT, alpha-1 antitrypsin; AAV, adeno-associated virus; AAV1, adeno-associated virus serotype 1; AAV1-LPL, adeno-associated virus serotype 1-lipoprotein lipase; AAV2, adeno-associated virus serotype 2; AAV2-rpe65, adeno-associated virus serotype 2 expressing RPE65 gene; AAV8-FIX, adeno-associated virus-2 expressing human factor IX; Ad5, adenovirus type 5; Ad-OTC, adenovirus-ornithine transcarbamylase deficiency; CF, cystic fibrosis; CRISPR/Cas9, clustered regularly interspaced short palindromic repeats-associated protein 9; GT, gene therapy; LPL, lipoprotein lipase; RNAi, RNA interference; RV, retrovirus; SCID-ADA, severe combined immunodeficiency-adenosine deaminase; TIL, tumor-infiltrating lymphocytes
Reference for timeline: Keeler AM et al. Clin Transl Sci. 2007;10:242-248. doi:10.1111/cts.12466