Applications of gene therapy in clinical trials
CNS-directed gene therapy
Gene therapy presents an opportunity to transform patients’ lives, and this is especially true for CNS diseases with high unmet need. A single gene therapy treatment may have the potential to offer long-lasting benefits. There are several different viruses, such as AAVs and lentiviruses, that can be used for CNS-directed gene therapy.5
In neurodegenerative diseases, choosing the best administration route is critical because of the impact of the blood brain barrier (BBB), which regulates the passage of molecules into the brain. To overcome this barrier, clinical trials have been designed such that the AAV-based gene therapy is administered directly into the brain tissue as a one-time dose. Real-time magnetic resonance imaging helps to guide catheter placement, monitor infusions, and provide early detection of intraoperative complications. While some therapies use direct brain injection, other delivery routes are also under investigation.
Huntington’s disease (HD)
In dominant genetic diseases with a gain of toxic function like HD, gene therapy strategies involve reducing mutant gene expression and blocking a disease-causing effect in cells.6 For HD, the therapeutic goal is to inhibit the production of the mutant protein, mHTT. AAV-based gene therapy that has been modified to express micro RNA (miRNA) for non-selective knockdown of the huntingtin gene, may represent a highly innovative and promising approach to treating HD in the future.7
STEP 17:
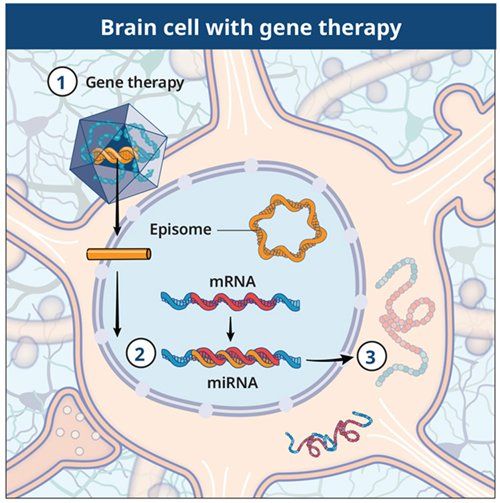
Entering the brain cell
The gene therapy will be directly infused into the brain under real-time magnetic resonance imaging guidance.
STEP 27:
Making blocking RNA (micro-RNA)
The gene therapy is intended to allow the brain cells to produce blocking RNA (micro-RNA) that interferes with huntingtin messenger RNA.
STEP 37:
Reducing the production of mutant huntingtin protein
This process is intended to reduce the production of mutant huntingtin protein.
Liver-directed gene therapy
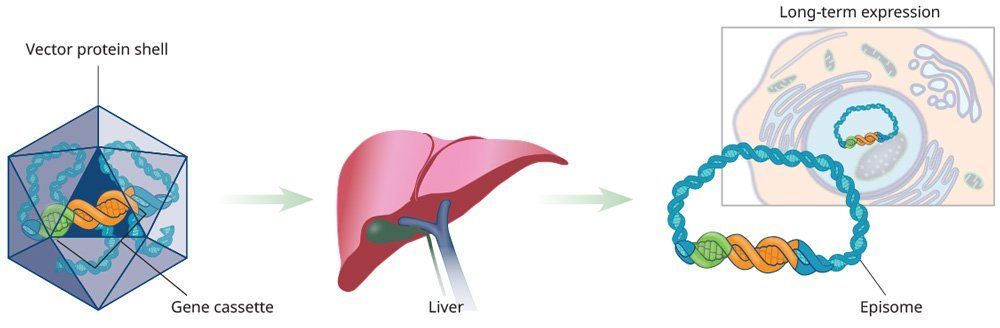
The discovery of different AAV protein shells and cellular tropism represents a well-designed and useful approach to tissue-specific, liver-directed gene therapy. Restricted expression can be achieved by using a tissue-specific promoter.8 Long-lasting gene expression depends, in part, on the type of promoter used to drive its expression.9 Tissue-specific promoters are advantageous because of their ability to direct expression of protein in target cells, while having no effect in other cell types.10,11 In investigational studies, liver-directed gene therapy has typically been administered through intravenous infusion.
Hemophilia
In X-linked recessive diseases like
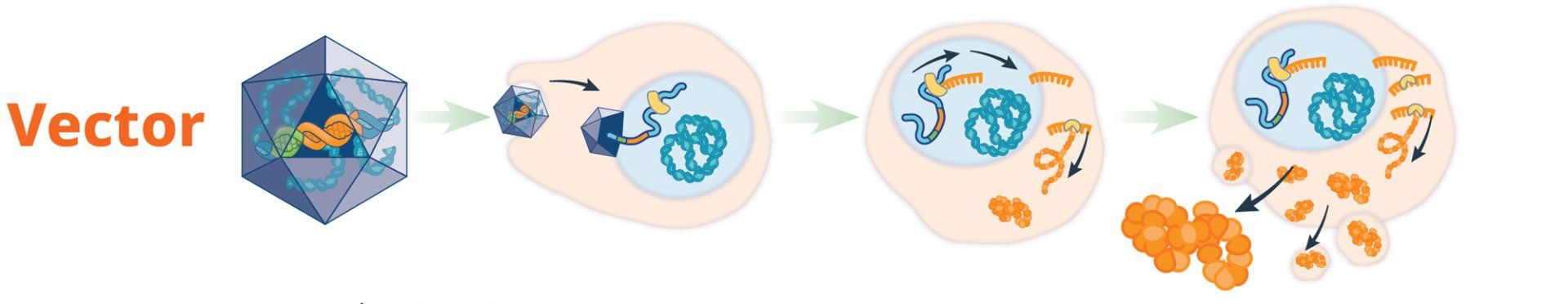
hemophilia, gene therapy strategies involve the transfer of a functional copy of the mutated gene into the target cell. The
functional gene
uses cellular machinery to create the missing or dysfunctional protein, which has the potential to reverse the disease phenotype. Introducing a copy of the working gene into the cell could restore the presence and natural function of the blood-clotting factor and prevent bleeding.6
Clinical trials are underway to determine the safety and efficacy of AAV-based gene therapy in hemophilia A and B.