Basic principles of technology
Gene therapy manufacturing platform
Gene therapy manufacturing platform
Genetic components for AAV-based gene therapies have been integrated into the genome of insect or mammalian production cell lines using helper viruses as carriers.1,23
Insect cell lines
The
baculovirus
platform has been used in research for more than 30 years in a variety of approved therapeutics, including vaccines for influenza and human papillomavirus (HPV).24
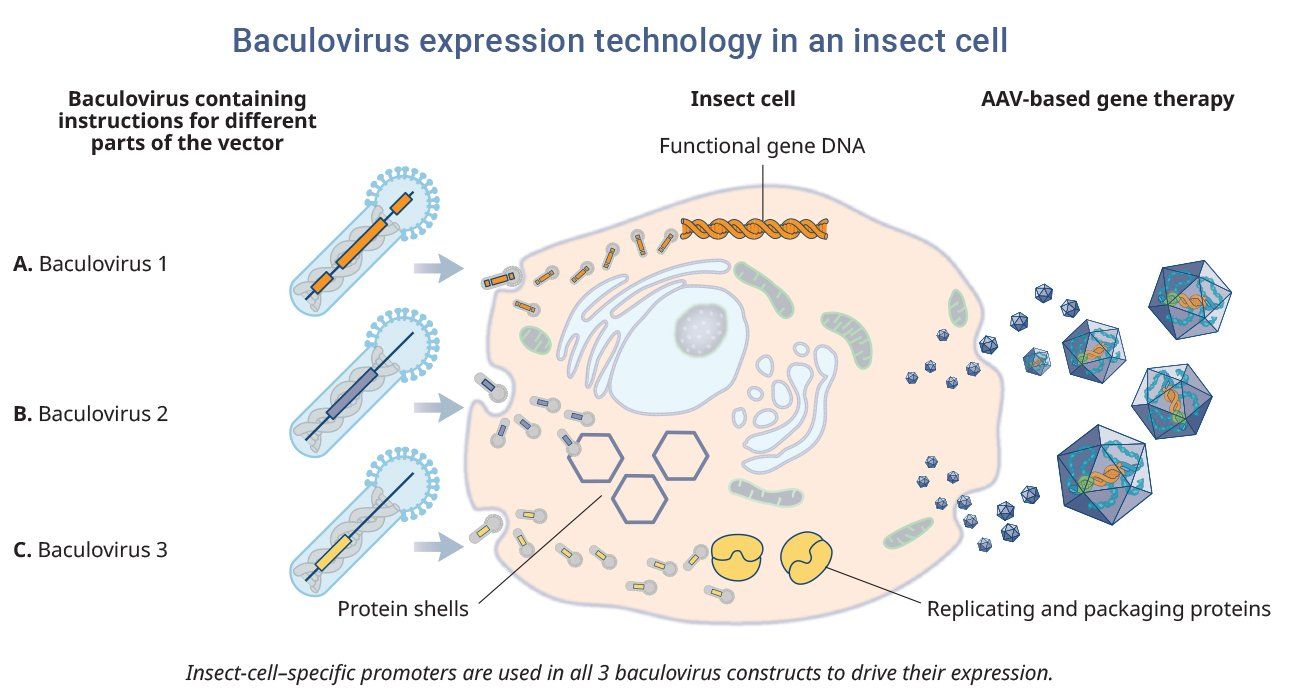
Baculoviruses are a common family of non-pathogenic viruses found in invertebrates and are used as carriers to co-infect insect cell lines. One of the advantages of the platform is that it offers a cost-effective, scalable method for producing high-quality products.25
Baculoviruses that contain instructions for the functional gene, protein shells, and proteins involved in replication and packaging are co-infected into insect cells. These instructions are then read by the insect cell, which assembles and produces the AAV-based gene therapy containing the desired gene cassette. Although there are no approved AAV-based gene therapy that use insect cell lines, there are FDA-approved vaccines that use the baculovirus platform.
Mammalian cell lines
Mammalian cells can also be used to produce AAV-based vectors. Mammalian cell lines may also use helper viruses, such as adenovirus or herpesvirus. However, there is ongoing research in development of AAV-based gene therapy in mammalian cell lines that are helper-virus free.1 There are two FDA-approved AAV gene therapies that use mammalian cell lines.26